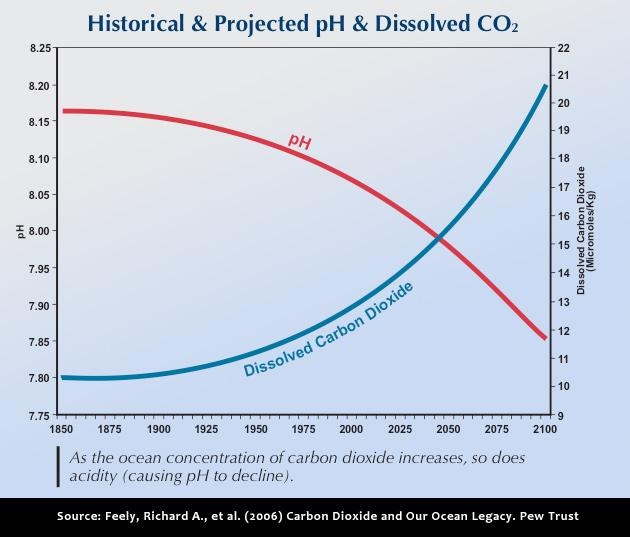
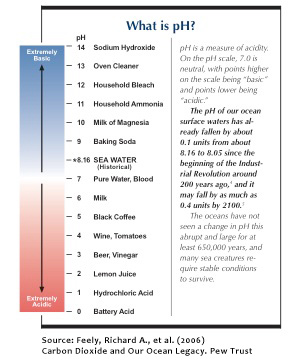
 The world’s oceans are currently under threat from a number of human impacts. On local scales, overfishing, deforestation and pollution from land can negatively affect marine ecosystems. On larger scales, global change and associated alterations in ocean circulation, increases in temperature and increases in storm intensity and frequency are all known to impact ocean life. One of the newest and perhaps most significant threats to marine ecosystems is Ocean Acidification (OA). OA is the result of a buildup of greenhouse gases — primarily carbon dioxide in the atmosphere. As these gases accumulate in the atmosphere, they also dissolve in the ocean causing oceanic pH to drop, making it more acidic.
The world’s oceans are currently under threat from a number of human impacts. On local scales, overfishing, deforestation and pollution from land can negatively affect marine ecosystems. On larger scales, global change and associated alterations in ocean circulation, increases in temperature and increases in storm intensity and frequency are all known to impact ocean life. One of the newest and perhaps most significant threats to marine ecosystems is Ocean Acidification (OA). OA is the result of a buildup of greenhouse gases — primarily carbon dioxide in the atmosphere. As these gases accumulate in the atmosphere, they also dissolve in the ocean causing oceanic pH to drop, making it more acidic.
The resulting ocean acidification can act like osteoporosis for marine organisms that build shells or have external skeletons such as abalone, mussels, oysters, corals and many others. ‘Fleshy’ organisms, such as kelp and other seaweeds, may be positively affected by ocean acidification because the increase in carbon dioxide acts like a fertilizer, resulting in increased growth rates.
The Smith lab is interested in understanding how ocean acidification will affect individual species, species’ interactions and benthic communities. We also believe it is important to monitor seawater chemistry locally, to understand the rates of change in our own ‘backyard.’ The Smith lab is therefore involved in ocean acidification monitoring, through the SOAR (Scripps Ocean Acidification Real-time) Monitoring Programand is continuously running ocean acidification experiments in the laboratory.